MTC-Y™
Semi-Solid Carrier
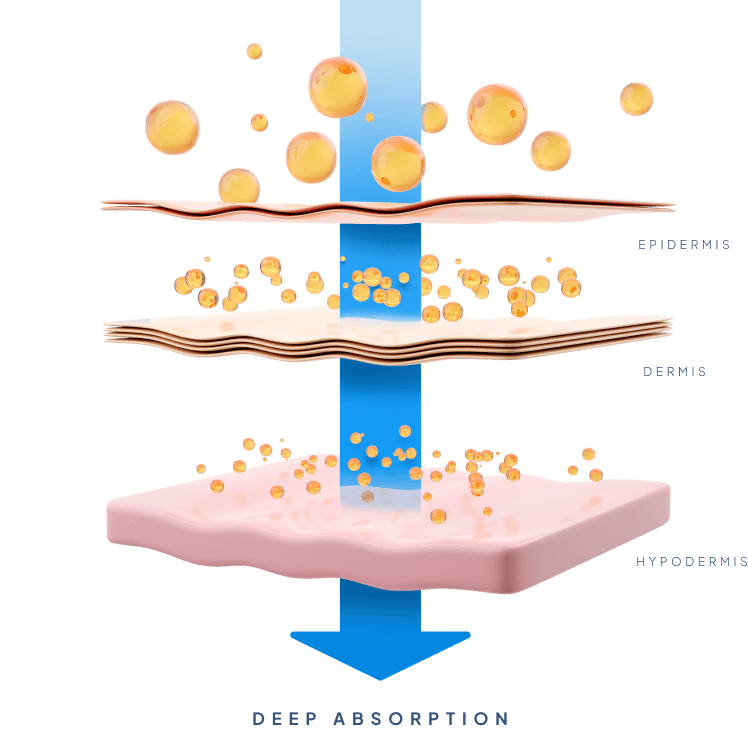
To be transdermal
Transdermal Drug Delivery has traditionally been limited by the skin’s tough and lipid rich outer layer known as the stratum corneum thus rendering the skin impermeable to most biopharmaceuticals; of the thousands of drugs currently approved by the FDA less than 30 are approved for transdermal delivery. So far, successful transdermal drug delivery has only been accomplished with small molecules (<500 Da.) that are moderately lipophilic. The development of Biotts’ proprietary MTC-Y™ technology has effectively removed these limitations, without the use of complicated devices or invasive technologies.
Biotts’ MTC-Y™ technology completely changes what is possible with Transdermal Drug Delivery, making it a much more feasible alternative. It offers the fast onset of action of an injectable, but at more constant and sustained drug levels and in a way that is much less invasive. Furthermore, it allows for reduced administration frequency and increased self-administration, and with that improved quality of life for patients. It also offers the convenience of an oral pill, but with a much more controlled and sustained release, and dramatically reduced side-effects and improved bioavailability.
Our technology
The mixture of excipients constituting the carrier of MTC-Y™ consists of components that ensure a simultaneous introduction of several active substances of different character and physicochemical properties into the body. Each of them can be introduced simultaneously in different polymorphic varieties, allowing for tailoring of the desired activity profile. It also enables significantly larger molecules – both lipophilic and hydrophilic – to penetrate through the skin barriers.
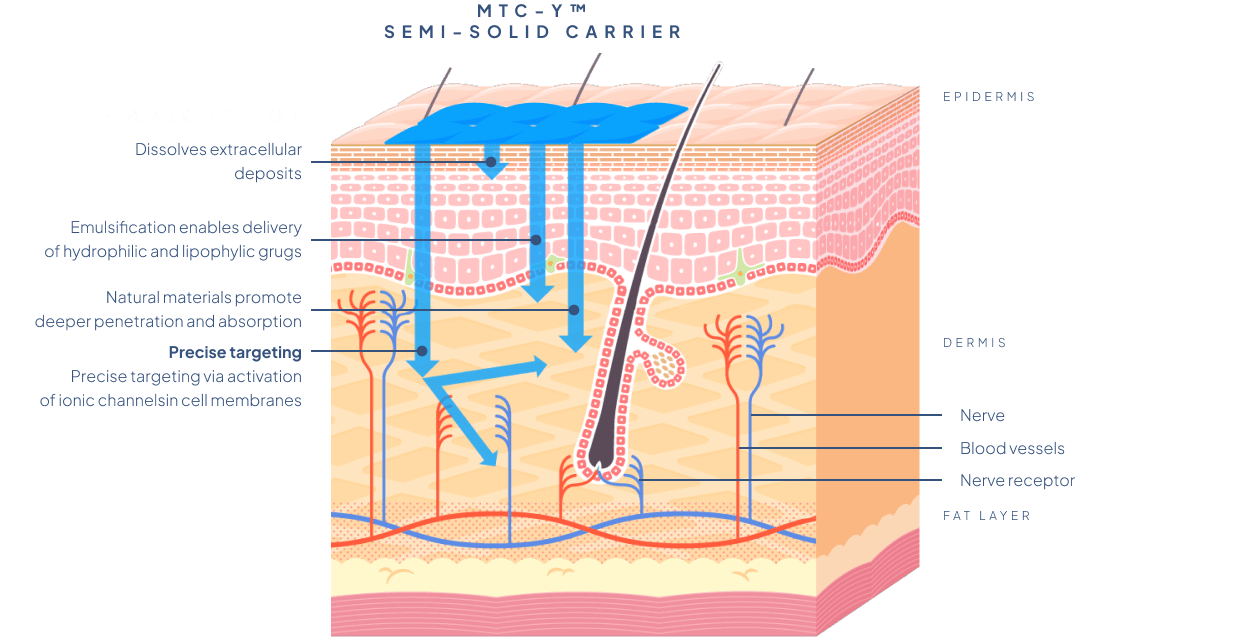
The MTC-Y™ Biotts system enables the transportation of one or more active substances, making it possilbe to design multi-component transdermal drugs. The MTC-Y™ carrier system is biocompatible with the human skin and non-toxic; it allows active ingredients to quickly penetrate the skin without irritating it.
particle [for now]
So far Biotts has generated Proof of Concept with a variety of molecules up to 6,000 Da. including several GLP-1 peptides like Liraglutide and Semaglutide, but there is no reason why we couldn’t go further. The possibilities of the MTC-Y™ technology seem endless.
First ever noninvasive insulin
Transdermal adminitration
In vivo PK study results – A comparison of Subcutaneous Injection to Transdermal Insulin Administration
Gottingen Mini pig study
Results
The study demonstrated the effectiveness of our transdermal insulin patch (MTC-INS) on a large animal model, comparing it to subcutaneous injection (SC).
The MTC-INS system achieved 70% of SC bioavailability, with both routes reaching peak concentrations (Cmax) at 6 hours. The transdermal administration showed sustained release, maintaining therapeutic levels longer.
Sprague Dawley rat study
Study design
The study was conducted using:
Insulin SC (Gensulin N – 0.035 mg/animal)
Transdermal MTC Carrier (insulin – 0.36 mg/animal)
The objective was to compare the insulin plasma concentration after subcutaneous administration to transdermal administration. Male Sprague Dawley rats were employed as the animal model for this study. Plasma insulin level was measured using the ELISA method.
Results
Bioavailability of transdermal insulin administration (MTC-I) was 55.1%.
MTC-I demonstrated a more sustained release over time than Subcutaneous administration (SC), maintaining higher concentration levels for a longer duration.
A single transdermal application of insulin using the MTC carrier can sustain therapeutic concentrations for up to 5 days.

The MTC-Y™ Carrier System study
Study design
The study was conducted using:
Subcutaneous injection (semaglutide – 0.3 mg/kg)
Biotts MTC-S1 ointment (semaglutide – 2.0 mg)
The study utilized rats as subjects and consisted of two groups. The first group received a single injection, while the second group had the ointment applied once, followed by occlusion for 3 days.
Results
Absolute bioavailability for MTC-S1 ointment was 4% – which is 5 to 10 times higher than current oral market solution.
Safety of the MTC-Y Carrier
clinically confirmed
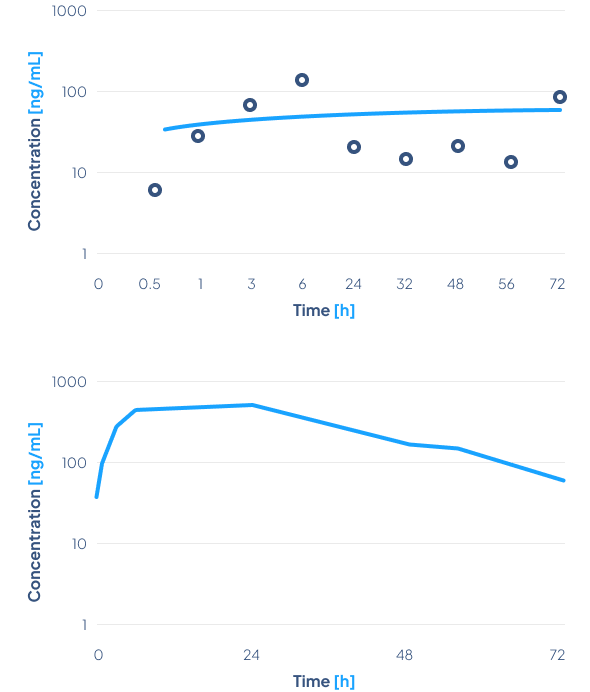
Comparison of oral and transdermal plasma of dapagliflozin concentration
Aim of the study
The primary objective of the phase 1 clinical study was to evaluate the safety, relative bioavailability and pharmacodynamic effect of a newly developed Dapagliflozin Transdermal System TTS MTC-D, after administration in healthy volunteers.
Results
Biotts’ transdermal TTS MTC-D system successfully delivered dapagliflozin through the skin and into the bloodstream.
The drug was well-tolerated, with mild side effects. The study confirmed the effectiveness of the MTC-Y carrier in delivering dapagliflozin, highlighting the potential of Biotts’ technology for diabetes treatment.
Scientific publications
Assessment of basic pharmacokinetic parameters of dapagliflozin in TTS formulations in male minipigs
Targeted action in the Synovial joint fluid
compared to the market standard
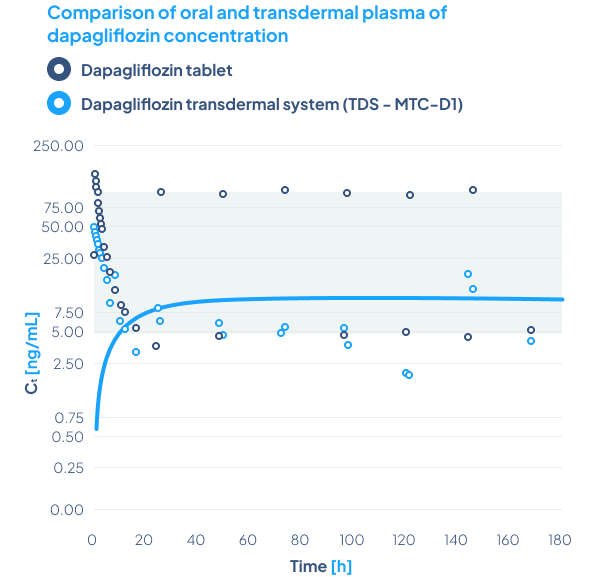
A comparison of topical administrated MTC-NL5 (Diclofenac) to Voltaren Max
Study design
The study was conducted using:
Biotts MTC-NL5 (diclofenac – 5.5 mg/ml)
Voltaren Max Gel (diclofenac – 22 mg/ml)
Male pigs were used for the study. The products were administered to the left leg only. A puncture of the knee joint was performed 1 hour after application, with prior cleaning of the puncture area.
Results
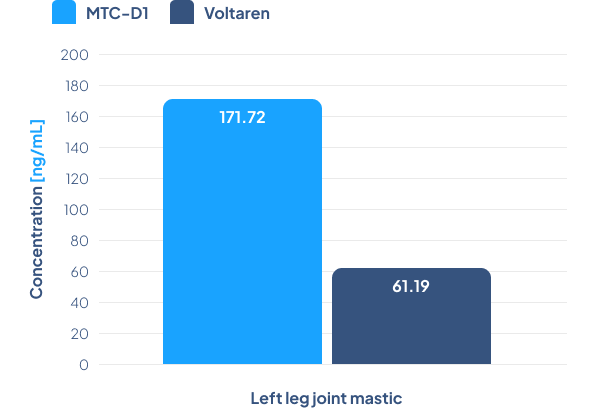
A 2.8-fold higher concentration of diclofenac was observed in the synovium of the left leg in animals treated with MTC-NL5 compared to animals treated with Voltaren Max Gel, meaning that the MTC-NL5 formulation is more than 11 times more effective than Voltaren Max Gel.
With the MTC-Y™ Carrier System
the treatment is controlled and sustained
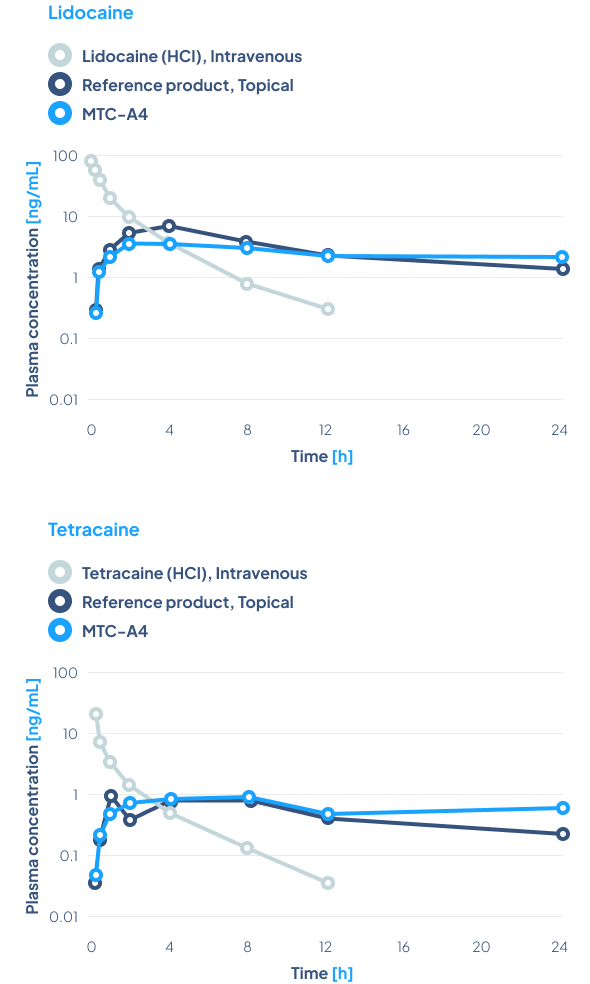
Study design
The concentration of the active substances, lidocaine and tetracaine, in the MTC-A4 product is 4% each, while in the reference product – Pliaglis – it is 7% each.
Results
The results show that using lower concentrations of active substances similar bioavailability of the same API was obtained. Furthermore, much more stable blood concentrations of the active substances released from MTC-A4 were obtained over a period of 24 hours, compared to the reference product.
Development collaborations
The MTC-Y™ carrier technology is the foundation of Biotts’ transdermal drug product development pipeline, but it’s also available to the pharmaceutical industry through contract development collaborations. Contact us or further information.