Transdermal
Drug Delivery Systems

MTC-Y™
Semi-Solid Carrier
Biotts is a biotech company specializing in Transdermal Drug Delivery
Our proprietary Multifunctional Transdermal Carrier Y (MTC-Y™) technology allows for the transdermal delivery of both lipophilic and hydrophilic molecules, that are much larger in size than traditional transdermal technologies, like peptides and proteins (up to 6000 Da. so far). This opens a whole new window of opportunity to the pharmaceutical industry and their patients to get the benefits from transdermal drug delivery like needle-free administration, sustained dosage, and increased bioavailability with a wide range of active ingredients.
Biotts develop its own transdermal drug products and make their expertise and technology available to the pharmaceutical industry through technical development collaborations.
Pushing the limits
Biotts is making
Transdermal Drug Delivery:
a viable alternative to oral
drugs and injectables
a benefit to improve
the quality of life
for millions of patients

Traditional
transdermal limitations
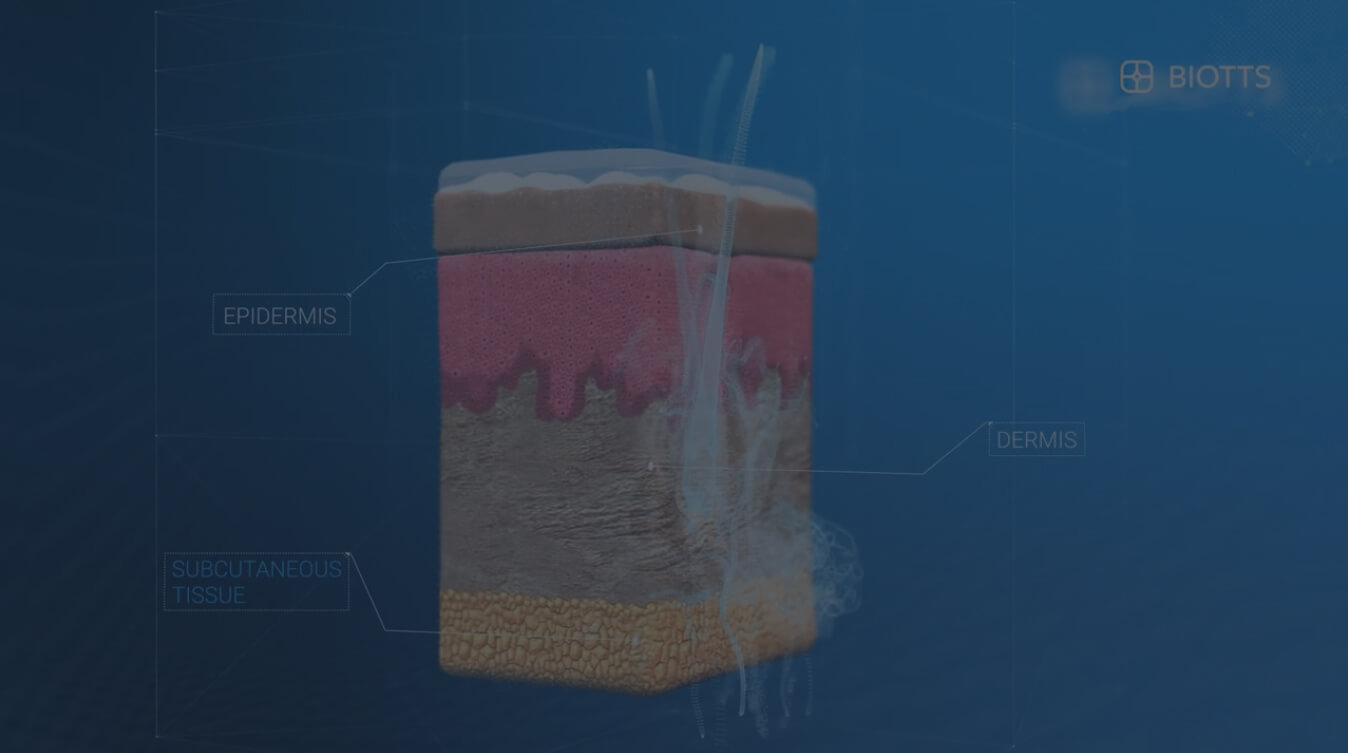
Transdermal Drug Delivery has traditionally been limited by the skin’s tough and lipid rich outer layer known as the stratum corneum thus rendering the skin impermeable to most biopharmaceuticals; of the thousands of drugs currently approved by the FDA less than 30 are approved for transdermal delivery. So far, successful transdermal drug delivery has only been accomplished with small molecules (< 500 Da.) that are moderately lipophilic.

Enhanced drug delivery
with MTC-Y™
The development of Biotts’ proprietary MTC-Y™ technology has effectively removed these limitations, without the use of complicated devices or invasive technologies.
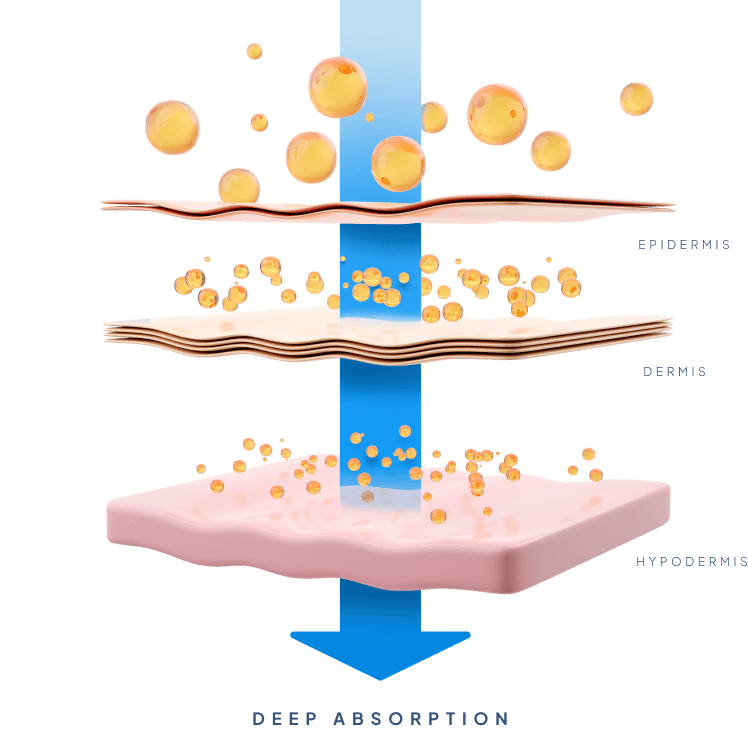
The MTC-Y™ carrier system is a semisolid emulsion that stimulates the transportation through the skin and can carry both lipophilic and hydrophilic molecules of up to 6,000 Da. such as peptides and proteins into the bloodstream. MTC-Y™ allows drugs to quickly penetrate the skin without irritating it.

Improving
patient outcomes
Biotts’ MTC-Y™ technology completely changes what is possible with Transdermal Drug Delivery, making it a much more feasible alternative.
It offers the fast onset of action of an injectable, but at more constant drug levels and in a way that is much less invasive. Furthermore, it allows for reduced administration frequency and increased self-administration, and with that improved quality of life for patients. It also offers the convenience of an oral pill, but with a much more controlled and sustained release profile, improved bioavailability, and dramatically reduced side-effects. The results are a significantly improved performance compared to existing transdermal and topical products, and unlimited opportunities for new transdermal product development.
patient outcomes