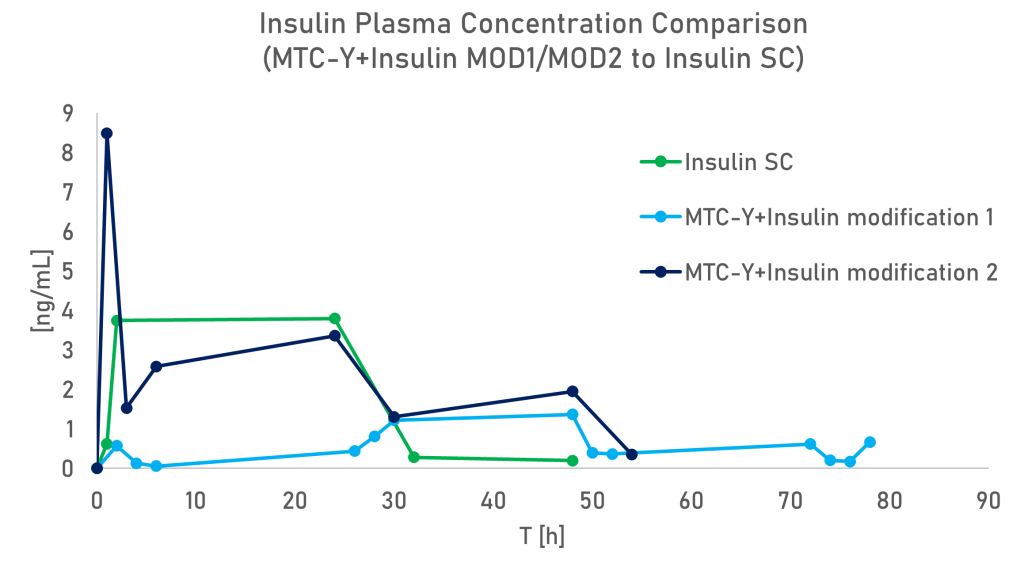
We’re excited to share our preclinical data from a rat study with you.
Study design
The study was conducted using:
Insulin SC (Gensulin N – 0.035 mg/animal)
MTC-Y+Insulin_MOD1 (insulin – 0.37 mg/animal)
MTC-Y+Insulin_MOD2 (insulin – 0.72 mg/animal)
The objective was to compare the insulin plasma concentration after subcutaneous administration to transdermal administration of both formulations of MTC-Y+Insulin Modifications.
Male Wistar rats were employed as the animal model for this study.
Results
This comparison demonstrates that insulin can be effectively administered transdermally without penetrating the layers of the skin.
Active pharmaceutical ingredient (API) concentrations achieved through transdermal administration are comparable to those attained through subcutaneous injection. Additionally, a single transdermal application of insulin using the MTC carrier can sustain therapeutic concentrations for up to 72 hours.